소개 소개: 효소 혁신에서 구조 생물학의 힘
인버타제와 같은 효소의 구조를 밝히는 것은 학문적 차원을 넘어 다음을 위한 관문입니다. 더 스마트하고 지속 가능한 솔루션. 통해 부위 지향적 돌연변이 유발 그리고 단백질 공학를 통해 효소의 안정성, 효율성 및 산업 적응성을 향상시키고 있습니다. 이러한 방법을 통해 천연 효소를 강력한 생체 촉매로 바꾸는 방법을 살펴보세요.
인버타제 구조 및 기능 이해
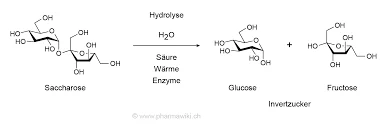
인버타제(β-프락토푸라노시다제)는 자당을 포도당과 과당으로 가수분해하는 효소입니다. 활성 부위 카르복실레이트 잔류물는 기질 인식 및 촉매 작용에 중요한 역할을 합니다. In 사카로미세스 세레비지애효소는 옥타메릭 4차 구조안정성과 기질 결합에 영향을 미치는 "개방형" 및 "폐쇄형" 이량체로 구성됩니다.
합리적인 부위 지향 돌연변이 유발: 최적의 기능을 위한 정밀 조정
연구자들은 열 안정성과 촉매 성능을 향상시키기 위해 잘 알려진 SUC2 인버타제를 표적으로 삼았습니다(PubMed 연구):
- P152V(Mut1)와 같은 돌연변이체 향상된 촉매 효율로 kcat/Km을 ~54%까지 향상시켰습니다.
- 결합된 치환(예: Mut4의 S305V/N463V)은 온도 안정성을 최대 16%까지 증가시켰습니다.
- 전략적 배치 소수성 잔류물 활성 사이트 루프에서 중요한 것으로 판명되었습니다.
재조합 발현 시스템: 코돈 최적화 및 호스트 선택
효율적인 재조합 발현이 핵심입니다:
- 피키아 파스토리스 는 유리한 활동성과 온도 안정성을 유지하여 최대 60°C의 최적의 활동을 유지합니다.
- 대장균 의 인버타제에서 볼 수 있듯이 코돈 컨텍스트 최적화를 통해 시스템의 이점을 얻을 수 있습니다. 써모토가 마리티마.
온도 안정성 향상: 분자 및 화학 공학
돌연변이 외에도 효소 안정화 방법에는 다음이 포함됩니다:
산업 회복탄력성을 위한 엔지니어링
성공 사례는 다음과 같습니다:
- 인버타제 엔지니어링 SDM 를 첨가하여 반죽 발효 활성을 최대 52%까지 향상시켰습니다.
- 온도 조절 인버타제 코돈 최적화 유전자 대장균*을 함유하고 있어 안정적으로 사용할 수 있습니다.
단백질 고정화: 재사용성 및 성능 향상
재조합 인버타제 고정화(예를 들어 지모모나스 모빌리스)를 나일론-6 비드에 붙입니다:
- 향상된 온도 안정성 - 30°C 및 70°C에서 더욱 안정된 50%(PubMed 소스).
- 내약성 pH ~5.5, 활동성 보존 - 연속 시스템에 이상적입니다.
산업 통합을 위한 실용적인 모범 사례
- 중요한 활성 부위 잔류물에 대한 효소 구조를 매핑합니다.
- SDM을 사용하여 촉매 사이트를 최적화하세요.
- 이상적인 표현 숙주를 선택합니다: *P. 파스토리스* 또는 *대장균*.
- 더 나은 발현을 위해 코돈을 최적화하여 유전자를 최적화하세요.
- 화학적/고정화 기술을 통해 효소를 안정화합니다.
- 복잡한 최적화를 위한 ML 가이드 진화를 살펴보세요.
파트너 BSTBIO 맞춤형 효소 솔루션
에서 BSTBIO, 우리의 인버타제 효소 오퍼링에는 다음이 포함됩니다:
- 고급 엔지니어링 지원 애플리케이션에 맞게 조정할 수 있습니다.
- 다음 옵션 돌연변이 디자인, 코돈 최적화 또는 고정화.
- 협업 R&D 를 통해 성능을 향상시키는 효소 솔루션을 제공합니다.
효소를 변화시킬 준비가 되셨나요?
연락처 BSTBIO 에:
- 정제된 샘플 또는 제형 준비가 완료된 샘플을 요청하세요.
- 돌연변이 유발 또는 고정화를 통해 맞춤형 인버타제를 설계하세요.
- 식품, 제약 또는 산업용 파일럿 협업에 대해 알아보세요.