Einleitung: Die Macht der Strukturbiologie bei der Enzyminnovation
Die Entschlüsselung der Struktur von Enzymen wie Invertase ist mehr als nur akademisch - sie ist ein Tor zur intelligentere, nachhaltige Lösungen. Über ortsgerichtete Mutagenese und Proteintechnikverbessern wir die Stabilität, Effizienz und industrielle Anpassungsfähigkeit von Enzymen. Sehen wir uns an, wie diese Methoden natürliche Enzyme in leistungsstarke Biokatalysatoren verwandeln.
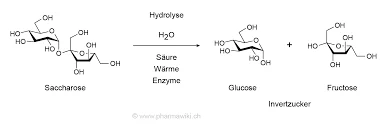
Struktur und Funktion der Invertase verstehen
Invertase (β-Fructofuranosidase) hydrolysiert Saccharose in Glucose und Fructose über ihre Carboxylat-Reste im aktiven Bereichund spielen eine entscheidende Rolle bei der Substraterkennung und Katalyse. Unter Saccharomyces cerevisiaebildet das Enzym eine oktamere quaternäre StrukturSie sind in "offene" und "geschlossene" Dimere organisiert, die die Stabilität und die Substratbindung beeinflussen.
Rationale ortsgerichtete Mutagenese: Präzise Korrekturen für optimale Funktion
Die Forscher zielten auf die bekannte SUC2-Invertase, um die Thermostabilität und die katalytische Leistung zu erhöhen (PubMed-Studie):
- Mutanten wie P152V (Mut1) verbesserte katalytische Effizienz - Erhöhung von kcat/Km um ~54%.
- Kombinierte Substitutionen (z. B. S305V/N463V in Mut4) erhöhten die Thermostabilität um ~16%.
- Strategische Platzierung von hydrophobe Rückstände an den Schleifen der aktiven Seite erwies sich als kritisch.
Rekombinante Expressionssysteme: Codon-Optimierung und Wirtswahl
Effiziente rekombinante Expression ist der Schlüssel:
- Pichia pastoris behält eine günstige Aktivität und Thermostabilität bei und behält bei ~60 °C seine optimale Aktivität.
- E. coli Systeme von der Codon-Kontext-Optimierung profitieren, wie bei Invertasen aus Thermotoga maritima.
Verbesserung der Thermostabilität: Molekular- und Chemietechnik
Neben Mutationen gibt es auch andere Methoden zur Stabilisierung von Enzymen:
- Chemische Quervernetzung mit Diisocyanaten verringerte die thermische Denaturierungsrate bei 60 °C um ~72%.
- Gezielte Entwicklungdie durch maschinelles Lernen verbessert werden kann, ergänzt das rationale Design.
Technik für industrielle Widerstandsfähigkeit
Zu den Erfolgsgeschichten gehören:
- Invertase, entwickelt über SDM zur Verbesserung der Gärungsaktivität des Teigs, die um ~52% erhöht wurde.
- Thermostabile Invertase aus kodon-optimierte Gene in *E. coli* für den robusten Einsatz.
Protein-Immobilisierung: Wiederverwendbarkeit und verbesserte Leistung
Immobilisierung rekombinanter Invertase (z. B. aus Zymomonas mobilis) auf Nylon-6-Perlen:
- Verbesserte Thermostabilität - 50% ist bei 30 °C und 70 °C stabiler (PubMed-Quelle).
- Verträglicher pH-Wert ~5,5 bei gleichbleibender Aktivität - ideal für kontinuierliche Systeme.
Praktische Best Practices für die industrielle Integration
- Kartierung der Enzymstruktur auf kritische Reste der aktiven Seite.
- Verwenden Sie SDM, um katalytische Stellen zu optimieren.
- Wählen Sie die idealen Expressionswirte: *P. pastoris* oder *E. coli*.
- Codon-Optimierung von Genen für eine bessere Expression.
- Stabilisierung von Enzymen durch chemische/Immobilisierungstechniken.
- Erforschen Sie die ML-geführte Evolution für komplexe Optimierungen.
Partner mit BSTBIO für maßgeschneiderte Enzymlösungen
Unter BSTBIOunser Enzym Invertase Das Angebot umfasst:
- Erweiterte technische Unterstützung zugeschnitten auf Ihre Anwendung.
- Optionen für Mutationsdesign, Codon-Optimierung oder Immobilisierung.
- Gemeinsame F&E um leistungssteigernde Enzymlösungen zu liefern.
Sind Sie bereit, Ihre Enzyme zu verändern?
Kontakt BSTBIO zu:
- Fordern Sie gereinigte oder formulierungsfertige Proben an.
- Entwicklung einer maßgeschneiderten Invertase durch Mutagenese oder Immobilisierung.
- Sondieren Sie eine Pilotkooperation für Lebensmittel, Pharmazeutika oder industrielle Anwendungen.